Background: Kawasaki disease (KD) is the leading cause of acquired heart disease in children in the developed world. It is an acute, generalized vasculitis, particularly in the coronary arteries, that occurs predominantly in infants and young children (Newburger et al, 2016). The acute phase of KD produces diffuse vascular inflammation that is thought to be associated with activation of the IL-1 pathway. Endothelium, located in the innermost layer of the coronary arteries, serves as an interface between the vascular media and circulating inflammatory cells and inflammatory cytokines. The coronary artery endothelium is the first target of the inflammatory attack in the early stages of KD (Qiu et al, Frontiers Cardiovascular Medicine, 2022) (Liu et al, Molecular Cell Biochem, 2021). There are pathologic changes during an acute arteritis with neutrophilic infiltration leading to necrosis in all the vessel walls, although the usual target is the medium sized extra-parenchymal muscular arteries. Current treatment is directed at reducing inflammation with IVIG and aspirin. Usually, this leads to rapid clinical improvement. However, 10-20% of patients are IVIG resistant and require further methods of reducing inflammation (Newburger et al, 2004). These patients that are IVIG resistant, have recrudescent fevers and evidence of ongoing inflammation, with subsequent increased risk for aneurysm formation (Tremoulet et al, 2008). Furthermore, other particular patient groups have been identified as having a higher risk (HR) of coronary artery aneurysms. Boys have a higher incidence of developing KD, but even accounting for that, are at a higher risk for developing coronary artery aneurysms (Belay et al, 2006). Defibrotide is a polydisperse mixture of predominantly single-stranded oligonucleotides (90% single-stranded and 10% double-stranded) derived from porcine intestinal mucosa. Several in vivo studies indicate that defibrotide primarily targets endothelium, particularly in small vessels, and appears to reduce endothelial cell injury and stabilize endothelial function (Falanga et al, Leukemia, 2003) (Cairo et al, BJH, 2020). Defibrotide is currently approved for SOS/VOD, with renal or pulmonary dysfunction post HSCT and has been well studied in infants with SOS post HSCT (Corbacioglu et al, Lancet, 2012). We hypothesized that Defibrotide will be safe and well tolerated in HR KD patients who fail IVIG and may be effective in difficult to treat KD patients, by mitigating ongoing endothelial dysfunction.
Primary Aims: To determine the safety and efficacy of defibrotide in infants with HR KD.
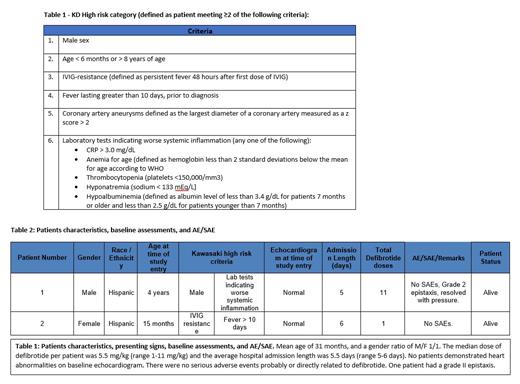
Design/Methods: Patients with KD as defined by the American Heart Association, have HR features (Table 1), age birth - 11 years of age, PT/PTT WNL, platelet count ≥100K/mm 3, and without active bleeding, hypersensitivity to defibrotide or receiving systemic anti-coagulant therapy, were eligible. Defibrotide, generously supplied by Jazz Pharmaceuticals, was administered within 96 hours of the conclusion of IVIG at 6.25 mg/kg IV Q6H (total daily dose 25 mg/kg/day) and continued for 7 days or until the patient was discharged from hospital, whichever occurred first (IND 127812) (NCT04777422). Stopping rules included: ≥ 15% grade III-IV allergic reaction or anaphylaxis or hemorrhage. All patients were treated with current standard of care, including: IVIG, aspirin (max 5mg/kg/dose), antibiotics, and intravenous fluids. Complete blood counts, coagulation studies and echocardiogram were performed at baseline, day 10, day 21, and 6 weeks after defibrotide was first given for disease assessment.
Results: We have preliminary enrolled 2 patients (mean age of 31 months, range 15 months to 4 years) with a gender ratio of M/F 1/1 (Table 2). The median dose of defibrotide per patient was 5.5 (range 1-11) and the average hospital admission length was 5.5 days (range 5-6 days). No patients demonstrated heart abnormalities on baseline echocardiogram. There were no serious adverse events (SAE) probably or directly related to defibrotide, as evaluated by investigators. One patient had a grade II epistaxis, but no patients had any SAEs.
Conclusion: This preliminary data suggests defibrotide is safe in children with HR KD failing IVIG. Further patients will be required to determine the safety and preliminary efficacy of defibrotide in children with HR KD. Supported in part by a research grant from Jazz Pharmaceuticals.
OffLabel Disclosure:
Cooke:Jazz Pharmaceuticals: Consultancy. Cairo:Amgen Inc.: Honoraria, Speakers Bureau; Servier Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy; Miltenyi Biotec: Research Funding; Sobi: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau; Astra Zeneca: Honoraria; Abbvie: Consultancy; Merck: Research Funding; Celularity: Research Funding; Omeros Pharmaceuticals: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Defibrotide

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal